REDUCING PATIENT EXPOSURE TO DEHP IN CLINICAL SETTINGS
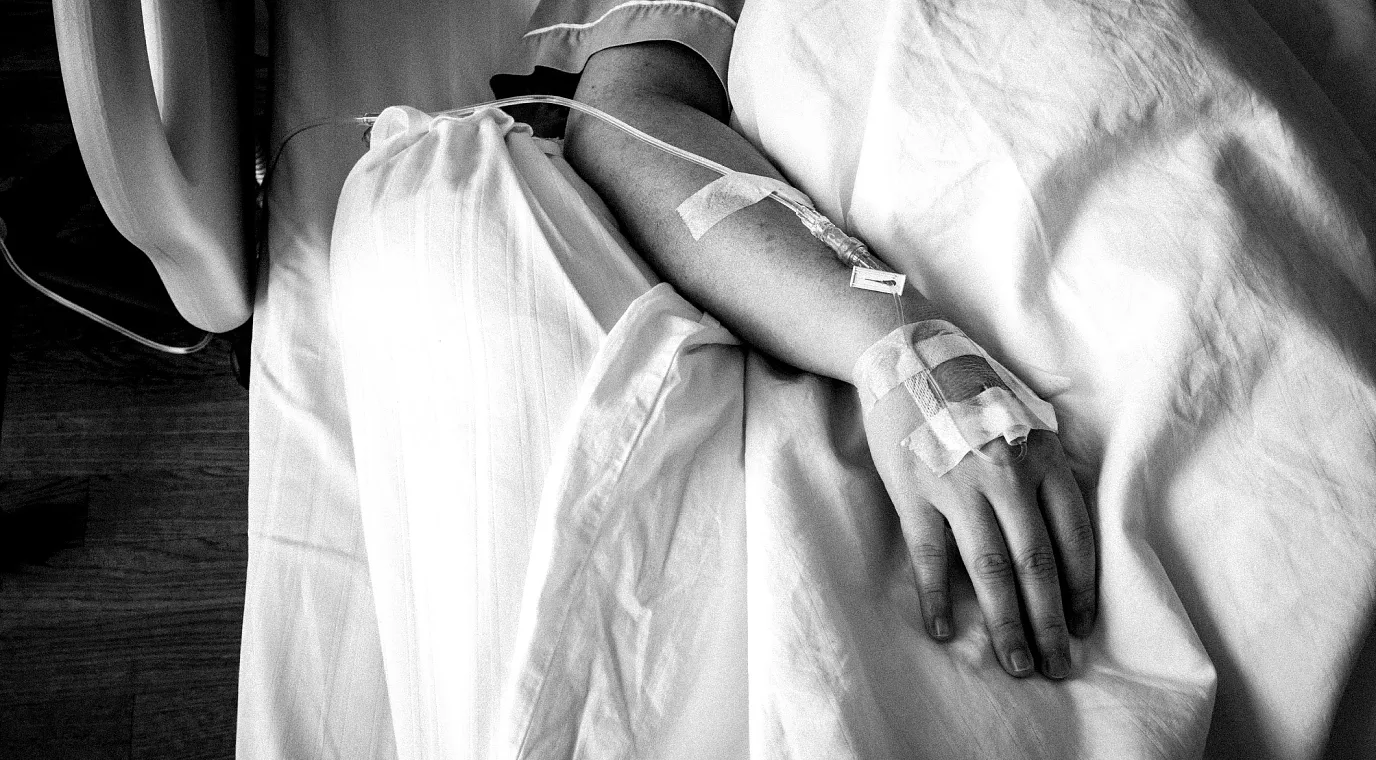
In clinical settings, you know that maintaining a safe and sterile environment is crucial for the safety of your patients—particularly at the point of care.
But here are some staggering statistics you may not know: Of the more than 33 million patients who are hospitalized in the U.S. every year, around 90% of them receive some form of IV treatment during their stay.1 And unfortunately, since 70% of IV bags contain di-(2-ethylhexyl) phthalate (DEHP),2 a majority of those patients are exposed to toxic chemicals without even knowing it.
According to the U.S. Environmental Protection Agency (EPA), pregnant women, fetuses, infants and young children may be the most vulnerable when it comes to the negative impact of toxic chemicals.3
So, what can you do as a healthcare provider to help limit patient exposure to toxic DEHP in products like IV bags and tubing at your facility?
In addition to signing a petition to the FDA on the Take Action page, here are a few more suggestions:
DEMAND CHANGE AT THE STATE AND FEDERAL LEVEL FOR PATIENT SAFETY
In 2024, California adopted the Toxic-Free Medical Devices Act, which bans the use of DEHP in IV bags beginning in 2030 and IV tubing in 2035. Similar legislation has been introduced in other states.
Contact your federal and state legislators to urge them to support legislation banning the use of DEHP in IV bags and tubing. Find your elected official: https://www.usa.gov/elected-officials
ADVOCATE FOR SAFETY POLICY CHANGES
While awareness at the state and national level is certainly growing, many hospitals and health systems nationwide are creating their own internal policies to protect patients from exposure to toxic chemicals. By following the "do no harm" bioethical principle of medicine, many have started creating policies to screen unsafe chemicals in the supply chain—before the products even get to their facility.3
PRIORITIZE HIGH-RISK PATIENTS
Despite the growing number of healthcare organizations that are proactively implementing their own internal policies to protect patients and staff, a recent study showed that hazardous chemicals can be found in at least 250 different products in the average hospital pediatric care room.3 Because we know these are some of our most vulnerable patients, we need to do more to make sure they're protected.
Toxic chemicals like DEHP are prevalent in many of our day-to-day products, not just in hospitals and healthcare facilities. But any steps healthcare providers can take—even small steps—to protect patients from harm should be explored. The fact that you're reading this article proves it's important to you.
Learn how you can take action to help improve hospital safety and urge the FDA to review and update federal guidelines on the use of DEHP in IV bags and tubing nationwide.
References: 1. Hammerand J. After pushing for DEHP ban, B. Braun offers tips on toxin’s removal from devices. Medical Design & Outsourcing. Published September 17, 2024. Accessed April 1, 2025. https://www.medicaldesignandoutsourcing.com/b-braun-dehp-pvc-toxic-devices-identify-remove-replace/ 2. B. Braun Data on File. 3. Lewis K, Nguyen M. Protecting high-risk patients from unsafe chemicals in the supply chain. Vizient Inc. Published 2021. Accessed April 1, 2025. https://vizientinc-delivery.sitecorecontenthub.cloud/api/public/content/b3b0d88d3baf4c2c8d60c0b482bde41c